
EicOsis Quarterly Newsletter
Dear Friends and Supporters,
This quarter marked a defining moment for EicOsis. With the launch of our collaboration with The Michael J. Fox Foundation (MJFF) to develop Parkinson’s disease as an indication, we are officially stepping into a broader role as a neuroinflammation company. While our foundation has been in pain management, this new partnership reinforces our commitment to addressing the unmet needs of patients suffering from neurodegenerative diseases.
In this newsletter, we are excited to share the story of how EicOsis has evolved into a leading CNS therapeutics company, advancing toward the clinic with groundbreaking science. We’ll also highlight our promising preclinical results across multiple indications, showcasing the future potential of our platform. As always, we’ll provide a snapshot of industry trends and a brief financial update.
Thank you for your continued support as we take this next step in shaping the future of neuroinflammation treatments.
IN THIS NEWSLETTER
Industry Analysis: Shifting Investment Trends in Q1 2025
The first quarter of 2025 has seen an overall increase in Biotech investment (Fig1) compared to Q1 2024, with total capital deployed rising by 6%.
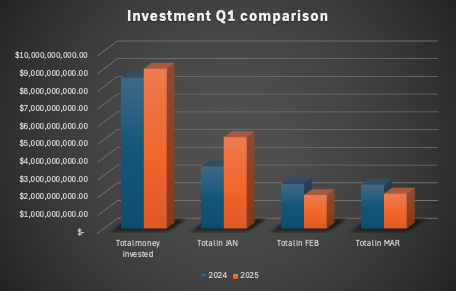
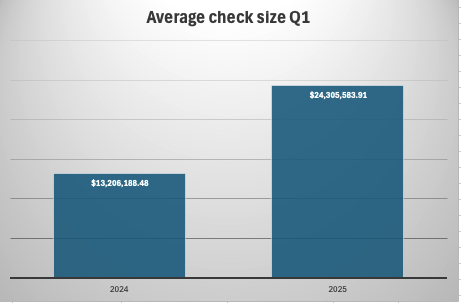
However, the number of deals has dropped significantly—by 42%—indicating a shift in investor behavior. With fewer deals being made, check sizes have increased dramatically, with the average transaction size nearly doubling (+84%) across all stages (Fig 2).
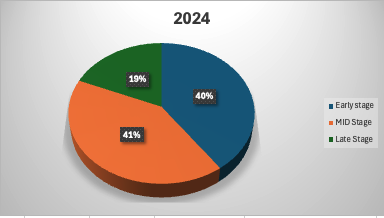
Early-stage investments have taken a slightly larger share of the market this year, growing by 26% compared to 2024. This suggests that investors remain interested in high-potential startups despite market turbulence. However, within early-stage funding, February saw a notable dip (-33%), reflecting ongoing volatility in investor sentiment. (Fig 3 + Fig 4)
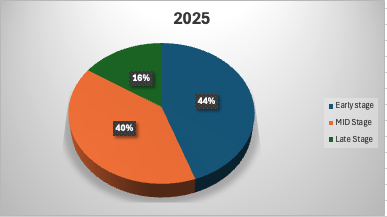
Mid-stage investments have also grown (+9%), with transaction sizes rising by 45%, signaling confidence in companies that have achieved meaningful milestones. Late-stage funding, while still strong, has shown mixed trends—while January saw a 29% increase, March saw a notable slowdown.
These shifts highlight changing market expectations and a preference for larger, more selective investments. Given this environment, our strategy for raising capital in 2025 should reflect these trends, potentially targeting a larger round to align with the market’s move toward bigger checks and fewer deals.
Financial Update: Stability Amid Industry Shifts
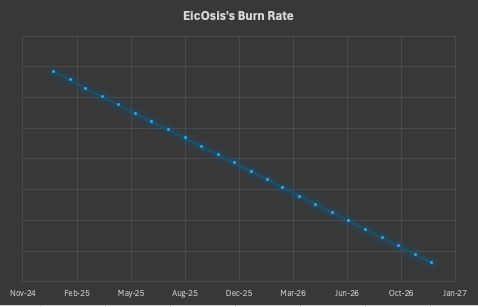
In a time of evolving policy changes, we want to reassure our stakeholders that EicOsis remains financially strong and well-positioned for the future. While recent headlines may suggest uncertainty in the biotech funding landscape, our foundation remains solid. Though we experienced a slight delay in grant reimbursements earlier this quarter, the issue has been resolved, and we are operating as usual.
Additionally, our recently announced collaboration with The Michael J. Fox Foundation (MJFF) is another key milestone, and importantly, their funding remains unaffected by recent policy shifts.
With our current funding and strategic planning, EicOsis has a stable two-year runway. At the same time, we are actively raising additional capital to ensure a seamless transition into Phase II clinical studies. We appreciate the continued support of our investors and partners as we advance our work in neuroinflammation and CNS therapeutics.
Our Science: From a Butterfly to PD

We are often asked about the significance of the butterfly on our outreach materials. In fact, EicOsis would likely not exist without a deep connection to insect biology. As a post-doctoral researcher, Bruce Hammock and his colleague, Sarjeett Gill, made an important early discovery about how caterpillars transform into butterflies. One of the enzymes responsible led to the discovery of the soluble epoxide hydrolase (sEH), our target enzyme in treating neuroinflammatory disease.
In his curiosity for understanding the biological role of this enzyme, he identified the enzyme in mammals as well as insects and realized that a similar biochemical pathway involving this enzyme plays a crucial role in regulating inflammation and pain in humans. This led to the development of sEH inhibitors, which EicOsis is now advancing as potential treatments for chronic pain, neuroinflammation, and cardiovascular diseases. His transition from studying insect development to human health is a remarkable example of how curiosity-driven research can lead to life-changing medical breakthroughs.


Future Indications
The incidences of both neurodegenerative diseases and chronic inflammatory conditions are on the rise, in part due to an aging population. In fact, neurodegenerative disorders, such as Parkinson’s Disease or Alzheimer’s Disease, are now the leading cause of disability in the world. For most of these conditions, there is a pressing unmet need for novel therapies that can alter disease progression. Neuroinflammation is a key process in the pathogenesis of neurodegenerative disorders but has often been overlooked as a potential therapeutic target. Inhibition of the soluble epoxide hydrolase (sEH) with EicOsis drug candidates plays a crucial role in neuroinflammation and neurodegenerative diseases by reducing harmful inflammatory mediators in the brain. In fact, inhibition of sEH has consistently shown neuroprotective effects in various animal models, including Alzheimer’s disease, Parkinson’s disease, ischemia-induced brain injury, and traumatic brain injury. Inhibiting sEH in these serious neurologic disorders does not only reduce neuroinflammation and other associated pathologic changes; it also has been shown to reduce cognitive impairment and have beneficial behavioral effects in these models. EicOsis is advancing its drug candidates for the treatment of neurodegenerative disorders, including Parkinson’s Disease and Alzheimer’s Disease. In addition, we have continued making exciting progress in clinical development for pain, and a new clinical trial just opened recruitment in Augusta, Georgia for patients suffering neuropathic pain from a traumatic spinal cord injury. We are also designing new clinical trials targeting populations where pain control has been elusive, specifically those with chronic kidney disease who cannot take any of the commonly available therapies for pain. Explore our promising pipeline, and make sure to subscribe to our newsletter to stay informed on the latest breakthroughs, updates, and partnership opportunities!
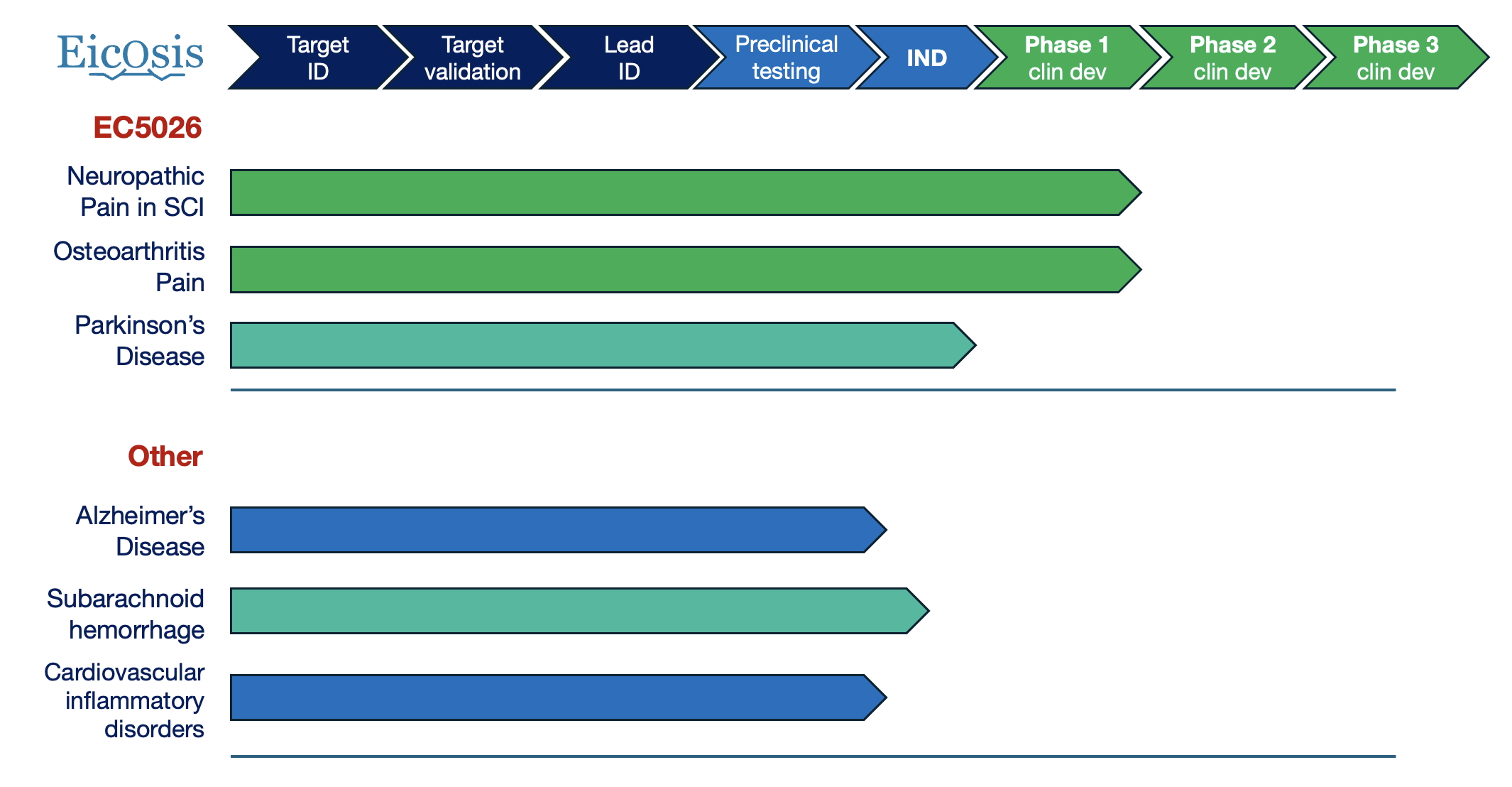
EicOsis is collaborating with leading experts to advance our drug candidates through clinical development as novel therapeutics for neuroinflammation. In collaboration with Dr. Lin Zhang, an expert in movement disorders and experimental therapeutics at the University of California Davis, and with support and funding from the Michael J Fox Foundation, EicOsis is planning to start a clinical trial of EC5026 in patients with Parkinson’s Disease later this year. This groundbreaking proof-of-concept clinical trial will provide key data to support future, larger, efficacy-focused clinical trials in Parkinson’s Disease. EicOsis is also working with experts in Alzheimer’s Disease, including Dr. Walter Swardfager from the University of Toronto, Canada, and Dr. Milan Fiala from UCLA.
Our Clinical Journey Continues
EicOsis recently opened recruitment for a clinical study to investigate pain in subjects with spinal cord injury (SCI). The clinical trial is being conducted in Augusta, Georgia with PI Dr. Laura Carbone.
The goal of this clinical trial is to evaluate safety and tolerability of multiple oral doses of EC5026 in male and female patients with neuropathic pain due to spinal cord injury. The main question it aims to answer is whether EC5026 is safe and well tolerated in SCI patients with neuropathic pain. In addition, this trial will also study the effects of EC5026 on pain.
Researchers will compare EC5026 to placebo (https://trialfinder.panfoundation.org/en-US/trial/listing/547475)
More information can be found at https://clinicaltrials.gov/study/NCT06438471
What’s next…
As we wrap up the first quarter of 2025, we remain steadfast in our commitment to advancing both our clinical programs and preclinical research. Our progress continues to build momentum, driven by promising data, strategic execution, and a dedicated team. With a stable financial outlook, we are well-positioned to expand our team, bringing in key talent to support the next phase of our growth.
We are especially excited about our collaboration with the Michael J. Fox Foundation (MJFF) and the impact it will have on our neuroinflammation research. Additionally, we are making significant progress toward onboarding our spinal cord injury clinical study in Georgia, an important milestone as we move forward in developing non-opioid pain solutions.
Looking ahead, we are preparing to raise a significant round toward the end of the year—one that will fuel our clinical advancements and accelerate our path to impact. We are also eager to announce new partnerships that are set to materialize later this year, strengthening our position in the industry. Stay tuned for more updates!
Thank you for your continued support and belief in our mission. Exciting developments lie ahead, and we look forward to sharing them with you in the coming months.
Contact us at info@eicosis.com.
Not on our mailing list?
Sign up to make sure you receive every update.


