
EicOsis Quarterly Newsletter
Dear EicOsis Community,
As we enter 2025, we’re excited to share the latest strides in our mission to revolutionize the treatment of neuroinflammatory diseases. The year 2024 has marked significant growth and achievement for EicOsis, highlighted by our recognition in Pepperdine University’s Most Fundable Companies list, a testament to our groundbreaking approach to pain management. Our lead compound is at the forefront of an evolving therapeutic landscape, with the potential to become a novel, safe, and effective treatment for neuroinflammation.
EicOsis’s unique mechanism, targeting the soluble epoxide hydrolase (sEH) enzyme, offers a new solution for conditions such as osteoarthritis, neuropathic pain due to spinal cord injury and diabetic neuropathy, and even neuroinflammatory conditions such as Parkinson’s Disease and Alzheimer’s Disease. Completing Phase 1 clinical trials in healthy volunteers demonstrated both safety and target engagement, setting the stage for upcoming clinical trials in patient populations. We are now raising funds to accelerate these studies, positioning us to bring this transformative therapy to those in need.
We invite you to stay connected as we move forward with these initiatives, leveraging our expanding IP portfolio and an exceptional team dedicated to addressing the unmet needs in pain management and inflammation. Thank you for being part of this journey toward a healthier, pain-free future.
IN THIS NEWSLETTER
- Our Industry: Investment in Pharma is booming. Learn why
- Our Science: Understanding more of the potential for sEH inhibitors in collaboration with the best research institutions. Explore more
- Our Progress: Tolerance, Pharmacokinetic, and safety details. Learn more about our journey
- Our Finance: Runway and fundraising updates. Get updated
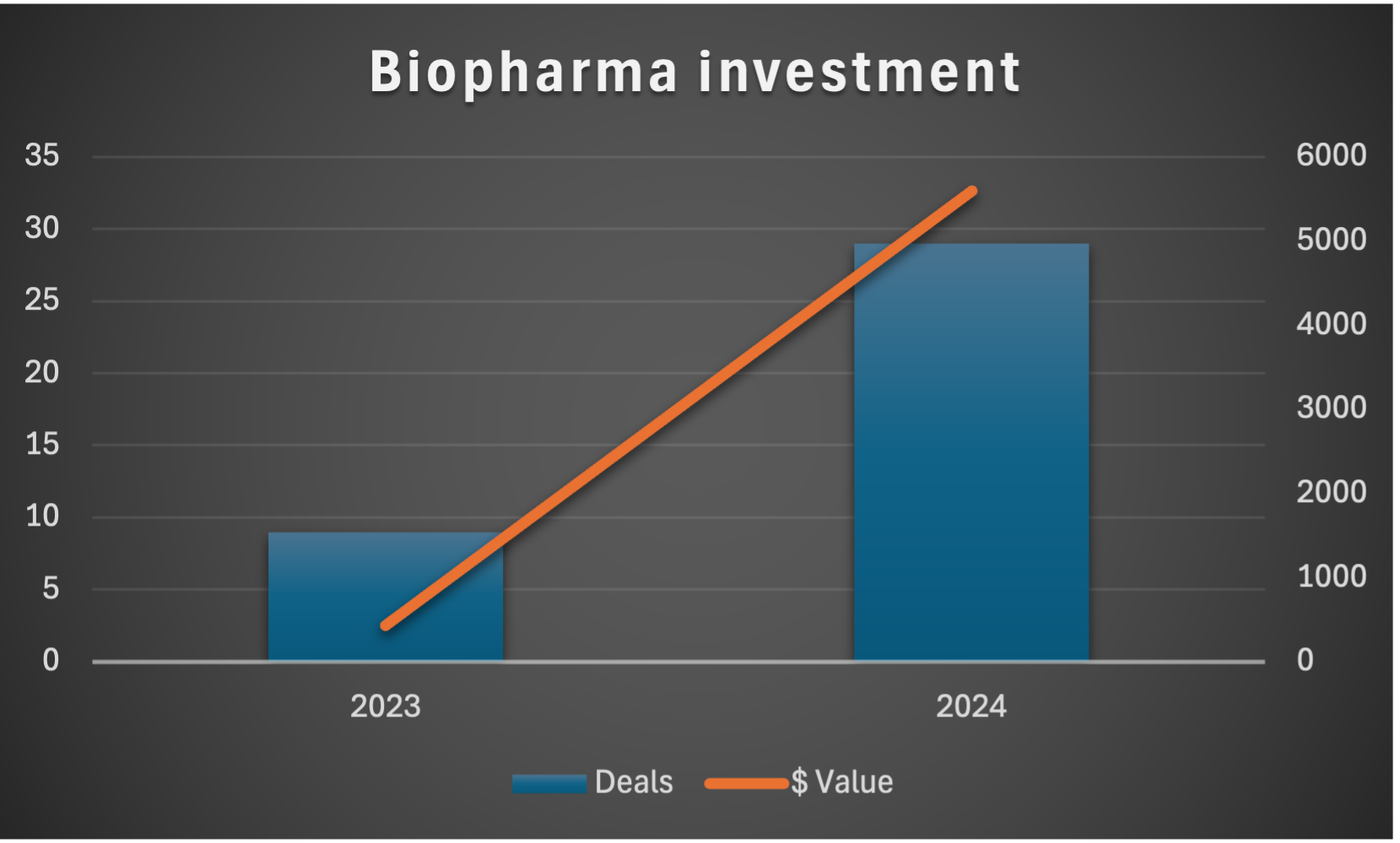
Industry Update: Why the Current Venture Environment is Ideal for EicOsis’ Funding Round
The neuroinflammation therapeutic space continues demonstrating robust growth and significant investor interest, making the current venture environment exceptionally favorable for EicOsis’ ongoing funding activities. Below is an analysis of recent deal trends that highlight why this is a promising moment for EicOsis:
1. Surging Deal Activity
In 2024, the number of deals in the neuroinflammation sector has more than tripled compared to 2023, with 29 deals announced or completed this year compared to only 9 in the prior year.
Notable investors in the sector include Andreessen Horowitz, RA Capital, Amgen Ventures, and OrbiMed Advisors, showcasing a mix of venture capital, corporate ventures, and specialized healthcare funds.
2. Increasing Deal Values
The total disclosed deal values in 2024 surged to over $5.6 billion, a significant increase from $433 million in 2023. This underscores growing confidence in the commercial potential of neuroinflammatory therapies.
3. Momentum Towards Clinical Development
Deals for clinical Phase 1 and Phase 2-stage companies have attracted the highest valuations, reflecting investor confidence in projects progressing through early clinical milestones.
Our Scientific Progress
Evidence of the beneficial effects of inhibiting the sEH pathway keeps growing.
Our collaboration with the University of Nottingham continues to advance the understanding of sEH activity in osteoarthritis, and their recently published paper identified an association between sEH-derived oxylipins and increased arthritis risk two years after a knee injury. The sEH pathway was recently highlighted in a special section of the British Pharmacological Society featuring a presentation by CEO Cindy McReynolds.
In a groundbreaking study conducted with collaborators at Harvard Medical School, sEH inhibition enhanced the anti-tumor activity of state-of-the-art cancer immunotherapy by promoting the resolution of cancer-associated inflammation.
Work on understanding the role of sEH in neuroinflammation has continued with multiple publications in prestigious journals in the last few years. Most notably, increased sEH activity correlated with Parkinson’s and Alzheimer’s Disease, and sEH was recently identified as a key risk factor and therapeutic target for Alzheimer’s disease (DOI: 10.1136/jnnp-2023-331142). These findings were presented by our collaborators at the Alzheimer’s Association International Conference 2024, reporting increased sEH activity as a biomarker of AD pathogenesis.
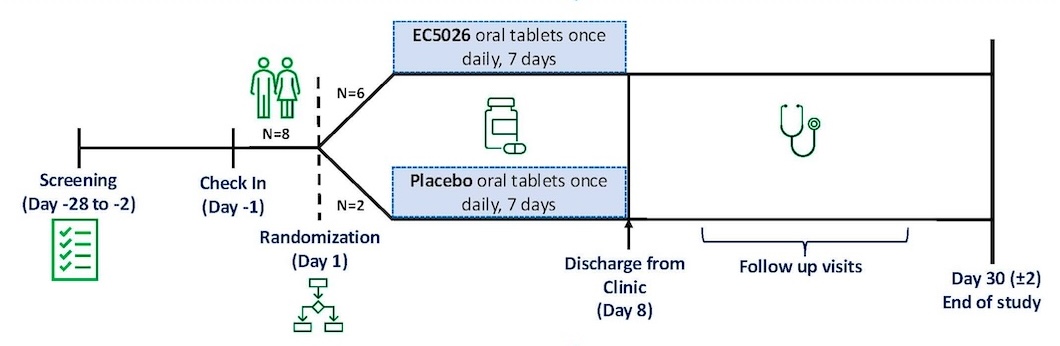
Our Clinical Progress
EicOsis is moving fast toward breakthrough solutions in pain management and neuroinflammatory diseases, and our momentum has never been stronger. We’ve just wrapped up a Phase 1b multiple ascending dose trial of our lead candidate, EC5026. The results? Incredibly promising. Across escalating dose regimens administered over a 7-day period, EC5026 was safe, well tolerated, and showed a favorable pharmacokinetic profile, paving the way for a convenient, once-daily dosing.
We recently published data from two earlier Phase 1a trials that reinforced these findings and demonstrated robust safety and bioavailability in single-dose and fed-fasted scenarios.
Now, we’re preparing to launch our first clinical trial targeting a specific pain condition early this year. In this exploratory study, participants with intractable neuropathic pain from spinal cord injury will be treated with EC5026 or placebo, allowing us to further assess safety, tolerability, and the potential impact of EC5026 on pain and function scales. EC5026 will be added on top of stable doses of existing neuropathic pain medications that have still left the participants with moderate-severe pain. The FDA has reviewed our protocol and the study site and investigator have been selected. This study will allow us to gather crucial data to inform the design of formal Phase 2 efficacy clinical trials.
With these achievements and our upcoming milestones, we are well-positioned at an inflection point. Now is the time to join us in transforming pain management through innovative therapies.

Our Financial Progress
EicOsis is proud to share a financial summary reflecting the company’s strong position and our strategic plans for the future. Our robust foundation is built on grant funding that provides the financial runway needed to advance our lead compound for the next two years. These non-dilutive resources enable us to focus on delivering impactful milestones in neuroinflammation and non-opioid pain relief while preserving equity value.
In parallel, we have initiated a SAFE Note, which has already demonstrated impressive traction among investors. This round will empower EicOsis to execute critical business initiatives. These include advancing our clinical programs, supporting a pre-IND for a new compound to extend our patent life, and making key hires. These actions will position the company to execute a Series A priced round in the second half of 2025.
Our lean operations have been instrumental in achieving our milestones efficiently and maintaining a clean and capable capital structure. The preclinical, clinical, and business results achieved in 2024 mark an inflection point for EicOsis, setting the stage for transformative growth.
As we continue to make strides in developing groundbreaking therapies for neuroinflammatory conditions, we remain committed to keeping our investors and collaborators informed about our progress. Thank you for your continued support as we advance toward these goals.
Our Next Milestones
- Preclinical data strongly support the potential benefit of sEH inhibitors in osteoarthritis, highlighting the promise of this therapeutic approach.
- In addition, EicOsis is advancing its Spinal Cord Injury (SCI) study development program, building on promising preclinical efficacy data.
- EicOsis is also broadening its scope by developing a new pipeline targeting neurologic diseases with an inflammatory component, such as Parkinson’s Disease, leveraging the anti-inflammatory potential of sEH inhibitors.
Plans for 2025 include initiating Phase 2 clinical trials for efficacy and safety, and expanding the clinical program team to support our growing pipeline.
Simultaneously, the company is advancing additional sEH inhibitors toward clinical development to expand its therapeutic offerings and address diverse unmet medical needs.
These milestones underscore EicOsis’s commitment to transforming care in pain and inflammation-driven conditions while building a robust and diversified clinical pipeline.
EicOsis is leveraging this unprecedented momentum within the neuroinflammation market. The company’s innovative approach, validated preclinical results, and progress toward Phase 2 clinical trials align perfectly with investor priorities.
We invite you to meet us at Biocom in San Diego in February to discuss this exciting opportunity in person. Additionally, for more information on the SAFE, please write to us at info@eicosis.com. Don’t miss the chance to be part of EicOsis’s transformative journey.
Not on our mailing list?
Sign up to make sure you receive every update.


