EC5026 Works by Increasing the Levels of Natural Analgesic and Anti-Inflammatory Molecules
EC5026 regulates the activity of powerful analgesic and anti-inflammatory fatty acids called epoxyeicosatrienoic acids (EETs) that are present in all cells in humans and animals. EC5026 inhibits the soluble epoxide hydrolase (sEH) enzyme, responsible of rapidly eliminating EETs. By inhibiting sEH, EC5026 increases the levels of EET levels at high anti-inflammatory and analgesic levels for 24 hours or longer.
Preclinical studies suggest that sEH inhibitors can decrease pain and inflammation and do not appear to produce sedation, cognitive dysfunction or other side effects common to currently available analgesics.
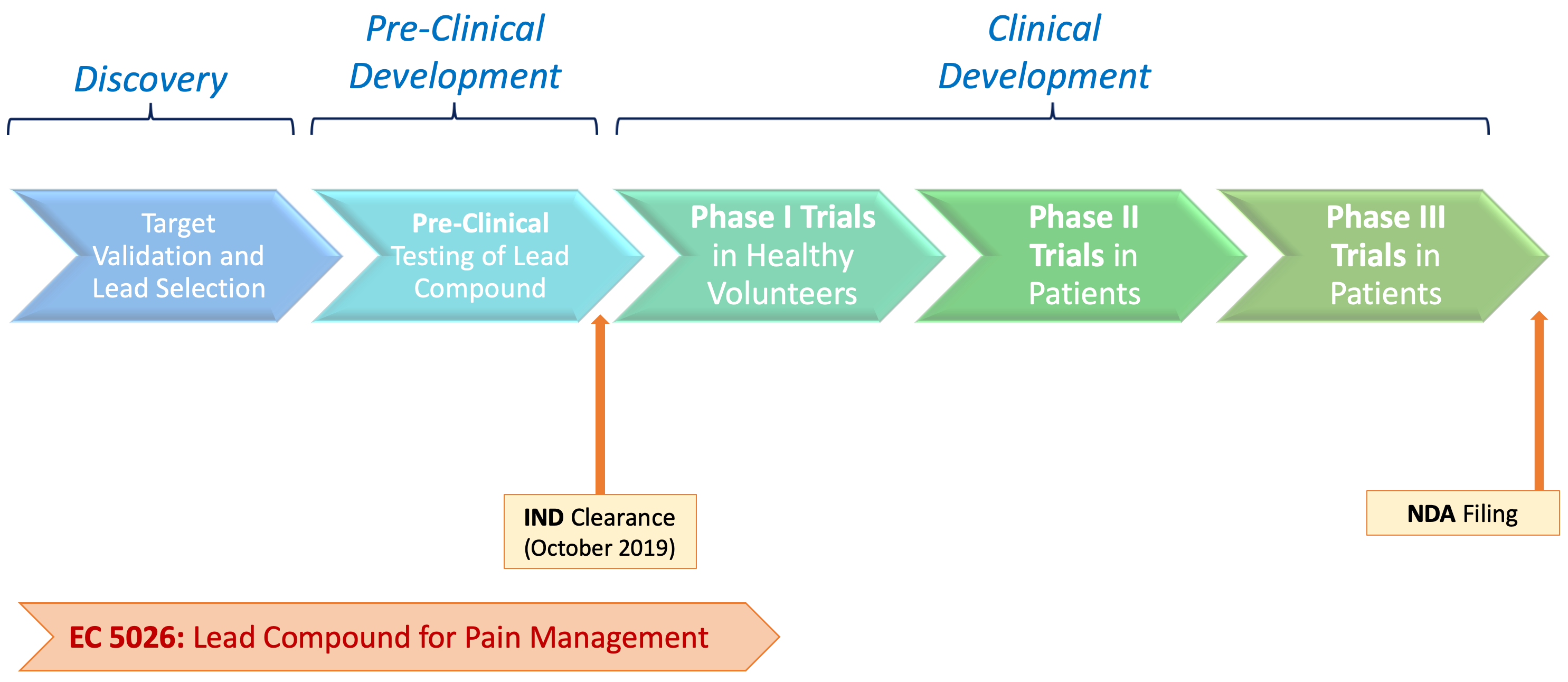
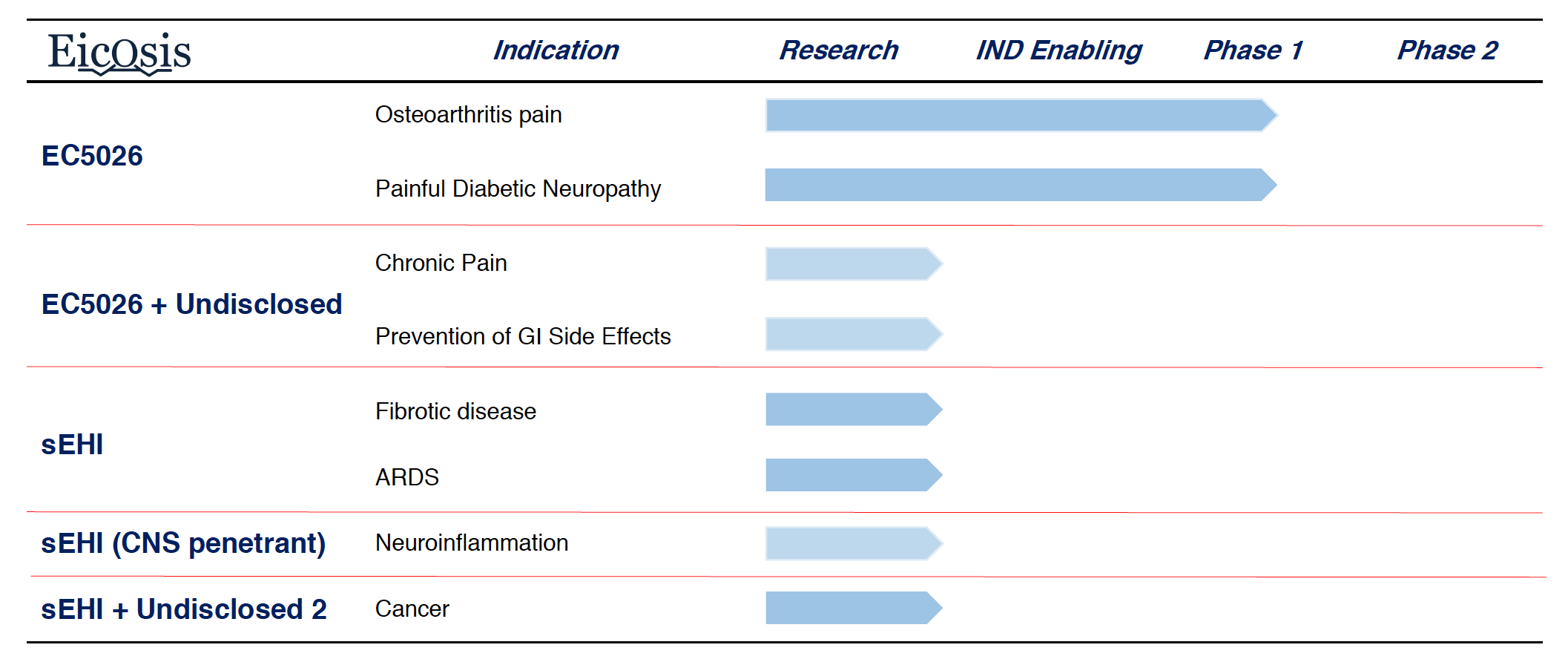
EC5026 is currently undergoing Phase 1 clinical trials
EicOsis received IND clearance from the FDA for EC5026 in October 2019. Phase 1a clinical trials in human volunteers started in December 2019, showing a favorable safety and pharmacokinetic profile, with no drug-related side effects. EicOsis is currently conducting additional Phase 1 clinical trials and is planning on evaluating EC5026 in pain patients soon.

Read about our Expanded Access Policy for EC5026.